2019: Remdesivir Drug Trial 1
Ebola Drug Trials
A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics
Remdesivir = 53.1% death rate
WHO & NIAID AWARE OF REMDESIVIR DEATH RATE
On 27 November 2019 results from the National Institutes of Heath (NIH), the Democratic Republic of Congo (DRC), and the World Health Organisation (WHO) clinical trial of 4 experimental drugs to treat ebola were published on the US National Institutes of Health website.
The trial known as PALM was coordinated by WHO, and led and funded by the DRC’s National Institute for Biomedical Research and Ministry of Health, and the National Institute of Allergy and Infectious Diseases (NIAID), part of the US National Institutes of Health.
The drug trials were undertaken from 2018 to 2019.
The purpose of the drug trials was to compare mortality.
Following the ebola drug trials, remdesivir was rejected for use to treat ebola.
Investigational Drugs Reduce Risk of Death from Ebola Virus Disease
The report, Investigational Drugs Reduce Risk of Death from Ebola Virus Disease, said:
“The study enrolled 681 people with Ebola virus disease between November 2018 and August 2019 at four Ebola treatment centers (ETCs) in the cities of Beni, Butembo, Katwa and Mangina.
The study was designed to compare mortality among patients who received one of three investigational Ebola drugs with that from a control group of patients who received the investigational monoclonal antibody cocktail ZMapp, developed by Mapp Biopharmaceutical, Inc.
The other therapies were mAb114, a single monoclonal antibody product developed for clinical use by NIAID’s Vaccine Research Center and the INRB and licensed to Ridgeback Biotherapeutics and Mapp Biopharmaceutical; REGN-EB3, a monoclonal antibody cocktail developed by Regeneron Pharmaceuticals, Inc.; and remdesivir, an antiviral drug developed by Gilead Sciences, Inc.
The Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Department of Health and Human Services, also has provided support for the development of REGN-EB3, ZMapp and mAb114.
The final analysis included 673 participants. The trial began in November 2018 with randomized administration of remdesivir, mAb114 and ZMapp, and the protocol was amended to include REGN-EB3 in January 2019. As a result, the number of outcomes included in the ZMapp comparator group was slightly different for the initial arms (remdesivir and mAb114) relative to the number of outcomes in the ZMapp comparator group for REGN-EB3, based on time of enrollment.
Overall mortality was 50% (84/169) in all patients treated with ZMapp and 51% (79/154) in patients who received ZMapp during the time that REGN-EB3 was included as another trial arm.
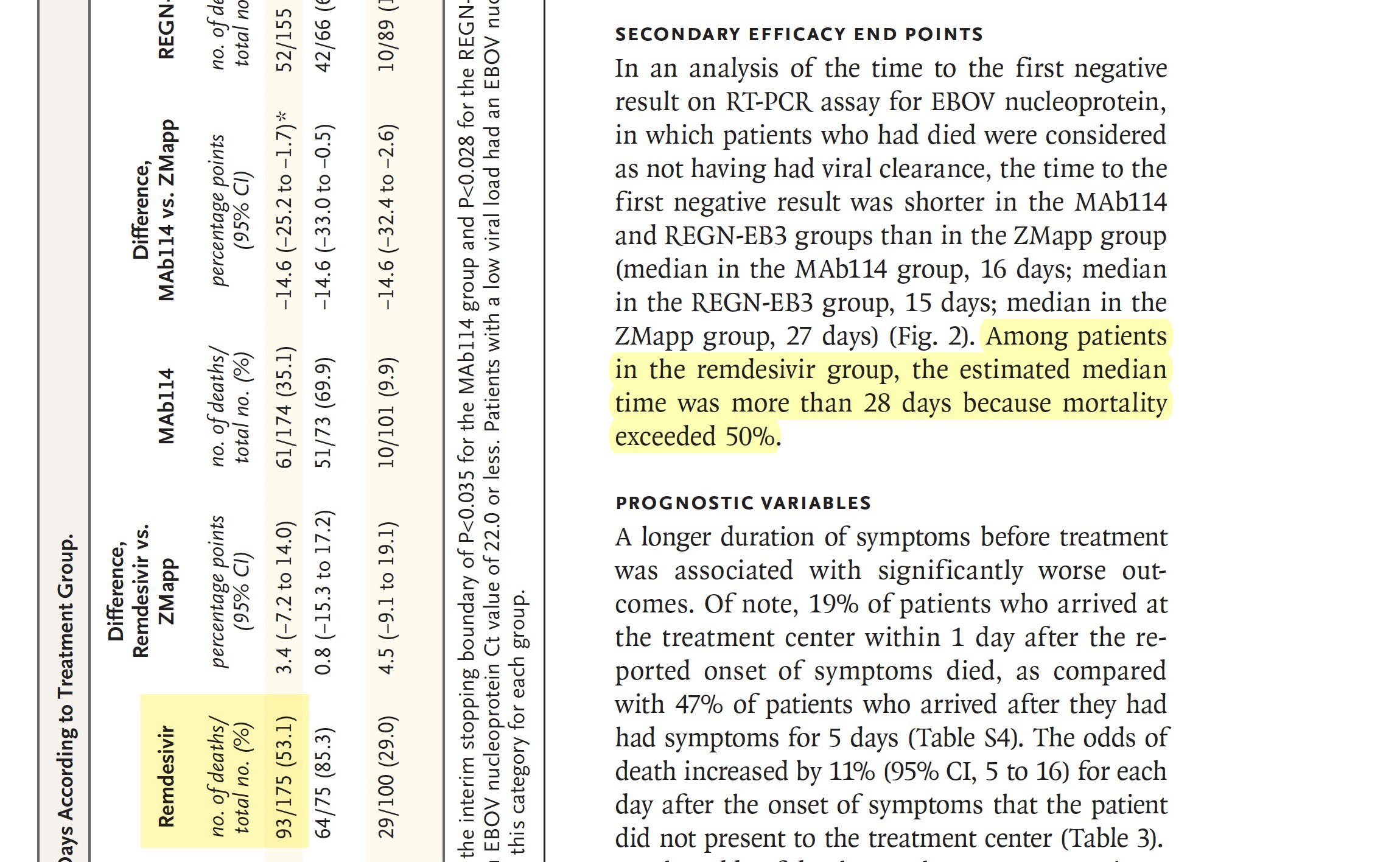
Mortality rates were lower for mAb114 and REGN-EB3 compared to their respective ZMapp cohorts: 35% (61/174) of patients in the mAb114 treatment group and 34% (52/155) of patients in the REGN-EB3 group died by 28 days post-treatment.
The mortality rate in the remdesivir treatment group, 53% (93/175), was similar to ZMapp.”
SNAPSHOTS OF RESULTS
Investigational Drugs Reduce Risk of Death from Ebola Virus Disease says,
The mortality rate in the remdesivir treatment group 53% (93/175).
Overall mortality rtes were lower in patients who had less virus in their blood at the time of enrolment… 29% for remdesivir.
The New England Journal of Medicine - A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics says,
Remdesivir no of deaths 93/175 (53.1).
Among patients in the remdesivir group, the estimated median time was more than 28 days because mortality exceeded 50%.
NIH Ebola Treatment Research says,
Remdesivir is no longer being administered to patients with Ebola virus disease in the DRC after the preliminary results of the PALM trial were announced.


The New England Journal of Medicine: Snapshot of Mortality Rate of Remdesivir
2019: Remdesivir Drug Trial 2
Drug Trial 2:
The journey of remdesivir: from Ebola to COVID-19
Drugs in Context reported on remdesivir results of the ebola drug trials, and on another drug trial undertaken in early 2020 for covid.
In this report, Drugs in Context provided results of:
Remdesivir for Ebola
Drugs in Context gives the results of remdesivir in the ebola trials undertaken in 2018 and 2019 and reports its mortality rate of 53.1%.Remdesivir for Covid
Drugs in Context gives results no results for remdesivir for the covid trial undertaken early 2020, reporting the trial was terminated on 15 April 2020.
A total of 53 patients were included in the trial however 8 patients were excluded on grounds of missing data. As such the trial is based on 45 patients.
The report does state that 7 of the 45 patients died, resulting in a mortality rate of 13% mortality.
It is important to note, the trial did not have any predefined endpoints or goals. In other words, the trial was not designed to assess mortality.
SNAPSHOTS OF RESULTS
Page 3 Remdesivir for Ebola says,
At day 28, mortality rates were remdesivir 53.1%.
For remdesivir 85 and 29% of patients with high and low viral loads at baseline died respectively.
Pages 4 & 5 Remdesivir for Covid says,
There were no predefined endpoints or enrolment goals. A total of 53 patients were included in the final analysis (8 patients were excluded due to missing or erroneous data).
Seven (13%) patients died.
The sample size was small, and a substantial portion of patients given remdesivir were eventually excluded from analysis.
Page 5 Remdesivir for Covid Table says,
Remdesivir for 10 days: April 15, 2020 Terminated.
Remdesivir for 10 days: April 15, 2020 Suspended.



2014 Plans for an Ebola Vaccine
NIAID DIRECTOR ANTHONY FAUCI ON EBOLA VACCINE
In 2014 the US National Institutes of Health stated clinical trials of an ebola vaccine would begin in 2014, with human testing possibly starting in 2015.
In the interview, Anthony Fauci said, “You want to balance the need to get a potentially effective vaccine to the people who need it as quickly as possible. At the same time, that you structure it in a way that you can get some meaningful information as to (a) whether it does work or not, and whether it does harm… We’ve had experience with vaccines that you actually think they will prevent infection and they make things worse.”
Fauci also said that those who are given the vaccine have to be monitored, and that vaccines are very different to providing experimental drugs to treat ebola. He said, “It’s not like a drug in which you a giving to a very sick person who has no other option, a vaccine is given to a healthy person. So the idea of do no harm is much much more compelling when you are dealing with someone who is a normal healthy person versus someone who is desperately ill and has no form of therapy but needs something.”
Fauci said, “We have an ongoing epidemic. We do not have a vaccine, so that’s a hypothetical. We do not have drugs, so that’s a hypothetical. Right now what we do have is infection control, isolation, quarantine and contact tracing. That is what is going to bring this epidemic under control.”
The World Health Organised asked for half a billion dollars to address this issue.





